What Is The Function Of The Rough Endoplasmic Reticulum In A Animal Cell
| Cell biology | |
|---|---|
| Animate being jail cell diagram | |
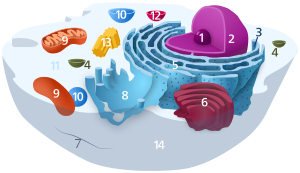 Components of a typical animal jail cell:
|

Micrograph of rough endoplasmic reticulum network around the nucleus (shown in the lower right-hand area of the moving picture). Night small circles in the network are mitochondria.
The endoplasmic reticulum (ER) is, in essence, the transportation arrangement of the eukaryotic cell, and has many other important functions such as protein folding. It is a blazon of organelle fabricated upward of two subunits – crude endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is institute in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not constitute in ruby claret cells, or spermatozoa.
The ii types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the jail cell. RER is constitute mainly toward the nucleus of cell and SER towards the prison cell membrane or plasma membrane of cell.
The outer (cytosolic) face of the RER is studded with ribosomes that are the sites of protein synthesis. The RER is peculiarly prominent in cells such as hepatocytes. The SER lacks ribosomes and functions in lipid synthesis but non metabolism, the production of steroid hormones, and detoxification.[1] The SER is specially abundant in mammalian liver and gonad cells.
The ER was observed with light microscope by Garnier in 1897, who coined the term ergastoplasm.[2] [3] With electron microscopy, the lacy membranes of the endoplasmic reticulum were first seen in 1945 by Keith R. Porter, Albert Claude, and Ernest F. Fullam.[4] Subsequently, the give-and-take reticulum, which means "network", was applied by Porter in 1953 to describe this textile of membranes.[five]
Structure [edit]
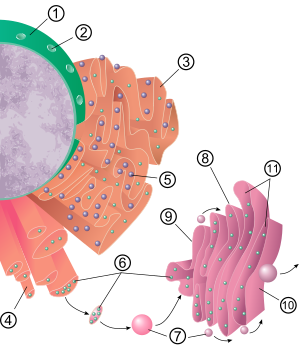
1 Nucleus 2 Nuclear pore iii Rough endoplasmic reticulum (RER) 4 Smoothen endoplasmic reticulum (SER) five Ribosome on the rough ER vi Proteins that are transported 7 Ship vesicle 8 Golgi apparatus 9 Cis face of the Golgi apparatus 10 Trans face up of the Golgi apparatus eleven Cisternae of the Golgi apparatus

3D rendering of endoplasmic reticulum
The general construction of the endoplasmic reticulum is a network of membranes chosen cisternae. These sac-like structures are held together past the cytoskeleton. The phospholipid membrane encloses the cisternal space (or lumen), which is continuous with the perinuclear space merely separate from the cytosol. The functions of the endoplasmic reticulum tin can exist summarized as the synthesis and export of proteins and membrane lipids, but varies betwixt ER and cell type and cell function. The quantity of both rough and smoothen endoplasmic reticulum in a cell tin slowly interchange from ane type to the other, depending on the changing metabolic activities of the cell. Transformation can include embedding of new proteins in membrane as well as structural changes. Changes in protein content may occur without noticeable structural changes.[vi] [7] [ citation needed ]
Rough endoplasmic reticulum [edit]

A 2-minute animation showing how a protein destined for the secretory pathway is synthesized into the rough endoplasmic reticulum, which appears at the upper right approximately halfway through the animation.
The surface of the crude endoplasmic reticulum (oft abbreviated RER or rough ER; also chosen granular endoplasmic reticulum) is studded with protein-manufacturing ribosomes giving it a "rough" advent (hence its name).[8] The bounden site of the ribosome on the rough endoplasmic reticulum is the translocon.[9] All the same, the ribosomes are not a stable part of this organelle'due south structure as they are constantly being bound and released from the membrane. A ribosome only binds to the RER once a specific protein-nucleic acid complex forms in the cytosol. This special complex forms when a free ribosome begins translating the mRNA of a poly peptide destined for the secretory pathway.[ten] The first v–30 amino acids polymerized encode a point peptide, a molecular message that is recognized and bound by a point recognition particle (SRP). Translation pauses and the ribosome complex binds to the RER translocon where translation continues with the nascent (new) protein forming into the RER lumen and/or membrane. The poly peptide is processed in the ER lumen past an enzyme (a point peptidase), which removes the signal peptide. Ribosomes at this point may be released back into the cytosol; even so, non-translating ribosomes are too known to stay associated with translocons.[eleven]
The membrane of the crude endoplasmic reticulum forms big double-membrane sheets that are located near, and continuous with, the outer layer of the nuclear envelope.[12] The double membrane sheets are stacked and continued through several right- or left-handed helical ramps, the "Terasaki ramps", giving ascension to a structure resembling a multi-story auto park.[thirteen] [fourteen] Although in that location is no continuous membrane betwixt the endoplasmic reticulum and the Golgi apparatus, membrane-leap transport vesicles shuttle proteins between these two compartments.[15] Vesicles are surrounded by coating proteins called COPI and COPII. COPII targets vesicles to the Golgi apparatus and COPI marks them to be brought back to the crude endoplasmic reticulum. The crude endoplasmic reticulum works in concert with the Golgi complex to target new proteins to their proper destinations. The 2d method of transport out of the endoplasmic reticulum involves areas called membrane contact sites, where the membranes of the endoplasmic reticulum and other organelles are held closely together, allowing the transfer of lipids and other small molecules.[sixteen] [17]
The rough endoplasmic reticulum is key in multiple functions:
- Manufacture of lysosomal enzymes with a mannose-six-phosphate mark added in the cis-Golgi network.[ citation needed ]
- Manufacture of secreted proteins, either secreted constitutively with no tag or secreted in a regulatory manner involving clathrin and paired basic amino acids in the betoken peptide.
- Integral membrane proteins that stay embedded in the membrane equally vesicles exit and bind to new membranes. Rab proteins are central in targeting the membrane; SNAP and SNARE proteins are primal in the fusion event.
- Initial glycosylation equally associates continues. This is N-linked (O-linking occurs in the Golgi).
- N-linked glycosylation: If the poly peptide is properly folded, oligosaccharyltransferase recognizes the AA sequence NXS or NXT (with the S/T residue phosphorylated) and adds a xiv-carbohydrate backbone (2-N-acetylglucosamine, 9-branching mannose, and iii-glucose at the terminate) to the side-chain nitrogen of Asn.
Shine endoplasmic reticulum [edit]

Electron micrograph showing smooth ER (arrow) in mouse tissue, at 110,510× magnification.
In most cells the smoothen endoplasmic reticulum (abbreviated SER) is scarce. Instead there are areas where the ER is partly smooth and partly rough, this area is called the transitional ER. The transitional ER gets its name because it contains ER exit sites. These are areas where the send vesicles that incorporate lipids and proteins made in the ER, detach from the ER and start moving to the Golgi apparatus. Specialized cells can have a lot of smooth endoplasmic reticulum and in these cells the smooth ER has many functions.[6] Information technology synthesizes lipids, phospholipids,[18] [19] [xx] and steroids. Cells which secrete these products, such as those in the testes, ovaries, and sebaceous glands have an affluence of smoothen endoplasmic reticulum.[21] It also carries out the metabolism of carbohydrates, detoxification of natural metabolism products and of alcohol and drugs, attachment of receptors on cell membrane proteins, and steroid metabolism.[22] In muscle cells, it regulates calcium ion concentration. Smooth endoplasmic reticulum is constitute in a variety of cell types (both brute and establish), and information technology serves dissimilar functions in each. The smooth endoplasmic reticulum also contains the enzyme glucose-6-phosphatase, which converts glucose-6-phosphate to glucose, a footstep in gluconeogenesis. Information technology is continued to the nuclear envelope and consists of tubules that are located near the cell periphery. These tubes sometimes branch forming a network that is reticular in appearance.[12] In some cells, there are dilated areas like the sacs of rough endoplasmic reticulum. The network of smoothen endoplasmic reticulum allows for an increased surface area to be devoted to the action or storage of central enzymes and the products of these enzymes.
Sarcoplasmic reticulum [edit]

The sarcoplasmic reticulum (SR), from the Greek σάρξ sarx ("mankind"), is smooth ER found in muscle cells. The only structural divergence betwixt this organelle and the polish endoplasmic reticulum is the medley of proteins they have, both bound to their membranes and drifting within the confines of their lumens. This fundamental difference is indicative of their functions: The endoplasmic reticulum synthesizes molecules, while the sarcoplasmic reticulum stores calcium ions and pumps them out into the sarcoplasm when the muscle fiber is stimulated.[23] [24] After their release from the sarcoplasmic reticulum, calcium ions collaborate with contractile proteins that utilize ATP to shorten the muscle cobweb. The sarcoplasmic reticulum plays a major role in excitation-contraction coupling.[25]
Functions [edit]
The endoplasmic reticulum serves many general functions, including the folding of poly peptide molecules in sacs called cisternae and the transport of synthesized proteins in vesicles to the Golgi apparatus. Crude endoplasmic reticulum is also involved in protein synthesis. Correct folding of newly made proteins is made possible by several endoplasmic reticulum chaperone proteins, including protein disulfide isomerase (PDI), ERp29, the Hsp70 family member BiP/Grp78, calnexin, calreticulin, and the peptidylprolyl isomerase family. Only properly folded proteins are transported from the rough ER to the Golgi apparatus – unfolded proteins cause an unfolded protein response as a stress response in the ER. Disturbances in redox regulation, calcium regulation, glucose deprivation, and viral infection[26] or the over-expression of proteins[27] can pb to endoplasmic reticulum stress response (ER stress), a state in which the folding of proteins slows, leading to an increase in unfolded proteins. This stress is emerging equally a potential cause of damage in hypoxia/ischemia, insulin resistance, and other disorders.[28]
Protein transport [edit]
Secretory proteins, generally glycoproteins, are moved beyond the endoplasmic reticulum membrane. Proteins that are transported by the endoplasmic reticulum throughout the cell are marked with an accost tag chosen a betoken sequence. The Northward-terminus (one end) of a polypeptide chain (i.e., a protein) contains a few amino acids that work every bit an address tag, which are removed when the polypeptide reaches its destination. Nascent peptides attain the ER via the translocon, a membrane-embedded multiprotein circuitous. Proteins that are destined for places outside the endoplasmic reticulum are packed into send vesicles and moved forth the cytoskeleton toward their destination. In human fibroblasts, the ER is e'er co-distributed with microtubules and the depolymerisation of the latter crusade its co-aggregation with mitochondria, which are as well associated with the ER.[29]
The endoplasmic reticulum is besides part of a protein sorting pathway. Information technology is, in essence, the transportation organization of the eukaryotic cell. The bulk of its resident proteins are retained within it through a retentivity motif. This motif is composed of 4 amino acids at the finish of the protein sequence. The well-nigh common retention sequences are KDEL for lumen located proteins and KKXX for transmembrane poly peptide.[30] However, variations of KDEL and KKXX do occur, and other sequences can as well give rise to endoplasmic reticulum retention. It is not known whether such variation can lead to sub-ER localizations. There are iii KDEL (one, 2 and 3) receptors in mammalian cells, and they have a very high degree of sequence identity. The functional differences betwixt these receptors remain to be established.[31]
Bioenergetics regulation of ER ATP supply by a CaATiER mechanism [edit]

Ca2+-antagonized transport into the endoplasmic reticulum (CaATiER) model
The endoplasmic reticulum does not harbor an ATP-regeneration machinery, and therefore requires ATP import from mitochondria. The imported ATP is vital for the ER to behave out its house keeping cellular functions, such every bit for protein folding and trafficking.[32]
The ER ATP transporter, SLC35B1/AXER, was recently cloned and characterized,[33] and the mitochondria supply ATP to the ER through a Caii+-antagonized send into the ER (CaATiER) mechanism.[34] The CaATiER mechanism shows sensitivity to cytosolic Ca2+ ranging from high nM to low μM range, with the Ca2+-sensing element yet to be identified and validated.
Clinical significance [edit]
Increased and supraphysiological ER stress in pancreatic β cells disrupts normal insulin secretion, leading to hyperinsulinemia[35] and consequently peripheral insulin resistance associated with obesity in humans.[36] Human clinical trials also suggested a causal link betwixt obesity-induced increase in insulin secretion and peripheral insulin resistance.[37]
Abnormalities in XBP1 lead to a heightened endoplasmic reticulum stress response and subsequently causes a higher susceptibility for inflammatory processes that may fifty-fifty contribute to Alzheimer's disease.[38] In the colon, XBP1 anomalies take been linked to the inflammatory bowel diseases including Crohn'southward disease.[39]
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum.[40] The UPR is activated in response to an aggregating of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum. The UPR functions to restore normal function of the cell by halting poly peptide translation, degrading misfolded proteins, and activating the signaling pathways that lead to increasing the product of molecular chaperones involved in protein folding. Sustained overactivation of the UPR has been implicated in prion diseases every bit well as several other neurodegenerative diseases and the inhibition of the UPR could become a treatment for those diseases.[41]
References [edit]
- ^ "Endoplasmic Reticulum (Rough and Smooth)". British Order of Prison cell Biology. Archived from the original on 24 November 2015. Retrieved 21 November 2015.
- ^ Garnier, C. (1897). "Les filaments basaux des cellules glandulaires. Annotation préliminaire". Bibliographie anatomique. 5: 278–289. OCLC 493441682.
- ^ Buvat, R. (1963). "Electron Microscopy of Plant Protoplasm". International Review of Cytology Volume fourteen. International Review of Cytology. Vol. 14. pp. 41–155. doi:10.1016/S0074-7696(08)60021-two. ISBN978-0-12-364314-8. PMID 14283576.
- ^ Porter KR, Claude A, Fullam EF (March 1945). "A study of tissue culture cells past electron microscopy: methods and preliminary observations". The Journal of Experimental Medicine. 81 (iii): 233–46. doi:10.1084/jem.81.3.233. PMC2135493. PMID 19871454.
- ^ PORTER KR (May 1953). "Observations on a submicroscopic basophilic component of cytoplasm". The Journal of Experimental Medicine. 97 (5): 727–fifty. doi:10.1084/jem.97.5.727. PMC2136295. PMID 13052830.
- ^ a b Alberts B, Johnson A, Lewis J, Raff 1000, Roberts M, Walter P (2002). Molecular biological science of the jail cell (4th ed.). New York: Garland Science. ISBN978-0-8153-3218-3. Archived from the original on 2017-10-03.
- ^ Cooper GM (2000). The cell: a molecular approach (2nd ed.). Washington (DC): ASM Press. ISBN978-0-87893-106-iv.
- ^ "reticulum". The Gratis Lexicon.
- ^ Görlich D, Prehn S, Hartmann Due east, Kalies KU, Rapoport TA (October 1992). "A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation". Cell. 71 (3): 489–503. doi:10.1016/0092-8674(92)90517-G. PMID 1423609. S2CID 19078317.
- ^ Lodish H, et al. (2003). Molecular Cell Biological science (5th ed.). W. H. Freeman. pp. 659–666. ISBN978-0-7167-4366-8.
- ^ Seiser RM, Nicchitta CV (October 2000). "The fate of membrane-bound ribosomes following the termination of protein synthesis". The Journal of Biological Chemistry. 275 (43): 33820–vii. doi:ten.1074/jbc.M004462200. PMID 10931837.
- ^ a b Shibata Y, Voeltz GK, Rapoport TA (August 2006). "Rough sheets and smooth tubules". Cell. 126 (three): 435–9. doi:10.1016/j.cell.2006.07.019. PMID 16901774. S2CID 16107069.
- ^ Terasaki 1000, Shemesh T, Kasthuri Due north, Klemm RW, Schalek R, Hayworth KJ, Hand AR, Yankova M, Huber G, Lichtman JW, Rapoport TA, Kozlov MM (July 2013). "Stacked endoplasmic reticulum sheets are continued by helicoidal membrane motifs". Cell. 154 (2): 285–96. doi:x.1016/j.cell.2013.06.031. PMC3767119. PMID 23870120.
- ^ Guven J, Huber G, Valencia DM (October 2014). "Terasaki spiral ramps in the rough endoplasmic reticulum". Physical Review Letters. 113 (18): 188101. Bibcode:2014PhRvL.113r8101G. doi:10.1103/PhysRevLett.113.188101. PMID 25396396.
- ^ Endoplasmic reticulum. (due north.d.). McGraw-Hill Encyclopedia of Science and Technology. Retrieved September xiii, 2006, from Answers.com Web site: "Answers - the Virtually Trusted Identify for Answering Life's Questions". Answers.com. Archived from the original on 2006-11-16. Retrieved 2006-09-xiii .
- ^ Levine T (September 2004). "Brusque-range intracellular trafficking of small molecules across endoplasmic reticulum junctions". Trends in Cell Biological science. xiv (9): 483–90. doi:x.1016/j.tcb.2004.07.017. PMID 15350976.
- ^ Levine T, Loewen C (Baronial 2006). "Inter-organelle membrane contact sites: through a glass, darkly". Current Stance in Prison cell Biology. 18 (four): 371–8. doi:10.1016/j.ceb.2006.06.011. PMID 16806880.
- ^ Kannan, Muthukumar; Lahiri, Sujoy; Liu, Li-Ka; Choudhary, Vineet; Prinz, William A. (March 2017). "Phosphatidylserine synthesis at membrane contact sites promotes its transport out of the ER". Periodical of Lipid Enquiry. 58 (three): 553–562. doi:x.1194/jlr.M072959. PMC5335585. PMID 28119445.
- ^ Kannan, Muthukumar; Riekhof, Wayne R.; Voelker, Dennis R. (February 2015). "Ship of Phosphatidylserine from the Endoplasmic Reticulum to the Site of Phosphatidylserine Decarboxylase2 in Yeast: Phosphatidylserine Transport to the Locus of Psd2p". Traffic. 16 (2): 123–134. doi:10.1111/tra.12236. PMID 25355612. S2CID 34302.
- ^ Friedman, Jonathan R.; Kannan, Muthukumar; Toulmay, Alexandre; Jan, Calvin H.; Weissman, Jonathan S.; Prinz, William A.; Nunnari, Jodi (January 2018). "Lipid Homeostasis Is Maintained by Dual Targeting of the Mitochondrial PE Biosynthesis Enzyme to the ER". Developmental Cell. 44 (2): 261–270.e6. doi:ten.1016/j.devcel.2017.11.023. PMC5975648. PMID 29290583.
- ^ "Functions of Smooth ER". University of Minnesota Duluth.
- ^ Maxfield FR, Wüstner D (October 2002). "Intracellular cholesterol send". The Periodical of Clinical Investigation. 110 (7): 891–eight. doi:10.1172/JCI16500. PMC151159. PMID 12370264.
- ^ Toyoshima C, Nakasako M, Nomura H, Ogawa H (June 2000). "Crystal structure of the calcium pump of sarcoplasmic reticulum at ii.6 A resolution". Nature. 405 (6787): 647–55. Bibcode:2000Natur.405..647T. doi:10.1038/35015017. PMID 10864315. S2CID 4316039.
- ^ Goodman SR (2007-11-26). Medical Cell Biology (tertiary ed.). Academic Press. p. 69. ISBN9780080919317.
- ^ Martini F, Nath J, Bartholomew E (2014). Fundamentals of Anatomy and Physiology (10th ed.). ISBN978-0321909077.
- ^ Xu C, Bailly-Maitre B, Reed JC (October 2005). "Endoplasmic reticulum stress: cell life and death decisions". The Journal of Clinical Investigation. 115 (10): 2656–64. doi:10.1172/JCI26373. PMC1236697. PMID 16200199.
- ^ Kober Fifty, Zehe C, Bode J (October 2012). "Development of a novel ER stress based selection organization for the isolation of highly productive clones". Biotechnology and Bioengineering. 109 (10): 2599–611. doi:x.1002/bit.24527. PMID 22510960. S2CID 25858120.
- ^ Ozcan U, Cao Q, Yilmaz East, Lee AH, Iwakoshi NN, Ozdelen East, Tuncman M, Görgün C, Glimcher LH, Hotamisligil GS (October 2004). "Endoplasmic reticulum stress links obesity, insulin action, and type two diabetes". Scientific discipline. 306 (5695): 457–61. Bibcode:2004Sci...306..457O. doi:x.1126/science.1103160. PMID 15486293. S2CID 22517395.
- ^ Soltys BJ, Gupta RS (1992). "Interrelationships of endoplasmic reticulum, mitochondria, intermediate filaments, and microtubules--a quadruple fluorescence labeling report". Biochemistry and Jail cell Biology. 70 (10–11): 1174–86. doi:10.1139/o92-163. PMID 1363623.
- ^ Stornaiuolo M, Lotti LV, Borgese N, Torrisi MR, Mottola G, Martire Thou, Bonatti S (March 2003). "KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex". Molecular Biology of the Cell. 14 (3): 889–902. doi:x.1091/mbc.E02-08-0468. PMC151567. PMID 12631711.
- ^ Raykhel I, Alanen H, Salo K, Jurvansuu J, Nguyen VD, Latva-Ranta M, Ruddock L (December 2007). "A molecular specificity code for the three mammalian KDEL receptors". The Journal of Cell Biology. 179 (half-dozen): 1193–204. doi:10.1083/jcb.200705180. PMC2140024. PMID 18086916.
- ^ Clairmont, CA; De Maio, A; Hirschberg, CB (25 February 1992). "Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94". The Journal of Biological Chemistry. 267 (six): 3983–90. doi:10.1016/S0021-9258(19)50622-6. PMID 1740446.
- ^ Klein, Marie-Christine; Zimmermann, Katharina; Schorr, Stefan; Landini, Martina; Klemens, Patrick A. Due west.; Altensell, Jacqueline; Jung, Martin; Krause, Elmar; Nguyen, Duy; Helms, Volkhard; Rettig, Jens; Fecher-Trost, Claudia; Cavalié, Adolfo; Hoth, Markus; Bogeski, Ivan; Neuhaus, H. Ekkehard; Zimmermann, Richard; Lang, Sven; Haferkamp, Ilka (28 Baronial 2018). "AXER is an ATP/ADP exchanger in the membrane of the endoplasmic reticulum". Nature Communications. 9 (one): 3489. Bibcode:2018NatCo...9.3489K. doi:10.1038/s41467-018-06003-9. PMC6113206. PMID 30154480.
- ^ Yong, Jing; Bischof, Helmut; Burgstaller, Sandra; Siirin, Marina; Spud, Anne; Malli, Roland; Kaufman, Randal J (9 September 2019). "Mitochondria supply ATP to the ER through a mechanism antagonized by cytosolic Ca2+". eLife. 8. doi:10.7554/eLife.49682. PMC6763289. PMID 31498082.
- ^ Yong, Jing; Johnson, James D.; Arvan, Peter; Han, Jaeseok; Kaufman, Randal J. (Baronial 2021). "Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus". Nature Reviews Endocrinology. 17 (viii): 455–467. doi:x.1038/s41574-021-00510-4. PMC8765009. PMID 34163039.
- ^ van Vliet, Stephan; Koh, Han-Grub E.; Patterson, Bruce W.; Yoshino, Mihoko; LaForest, Richard; Gropler, Robert J.; Klein, Samuel; Mittendorfer, Bettina (1 October 2020). "Obesity Is Associated With Increased Basal and Postprandial β-Cell Insulin Secretion Even in the Absence of Insulin Resistance". Diabetes. 69 (10): 2112–2119. doi:ten.2337/db20-0377. PMC7506835. PMID 32651241.
- ^ Mittendorfer, Bettina; Patterson, Bruce W.; Smith, Gordon I.; Yoshino, Mihoko; Klein, Samuel (1 February 2022). "β Cell function and plasma insulin clearance in people with obesity and different glycemic status". Journal of Clinical Investigation. 132 (3): e154068. doi:10.1172/JCI154068. PMC8803344. PMID 34905513.
- ^ Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez Chiliad, Rincon-Limas DE, Fernandez-Funez P (June 2011). "The ER stress gene XBP1s prevents amyloid-beta neurotoxicity". Human Molecular Genetics. 20 (11): 2144–60. doi:x.1093/hmg/ddr100. PMC3090193. PMID 21389082.
- ^ Kaser A, Lee AH, Franke A, Glickman JN, Zeissig Due south, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS (September 2008). "XBP1 links ER stress to intestinal inflammation and confers genetic chance for human inflammatory bowel disease". Cell. 134 (5): 743–56. doi:10.1016/j.jail cell.2008.07.021. PMC2586148. PMID 18775308.
- ^ Walter, Peter. "Peter Walter's Short Talk: Unfolding the UPR". iBiology.
- ^ Moreno JA, Halliday M, Molloy C, Radford H, Verity Due north, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR (October 2013). "Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical affliction in prion-infected mice". Scientific discipline Translational Medicine. 5 (206): 206ra138. doi:10.1126/scitranslmed.3006767. PMID 24107777. S2CID 25570626.
External links [edit]
- Endoplasmic Reticulum
- Lipid and poly peptide composition of Endoplasmic reticulum in OPM database
- Animations of the various cell functions referenced here Archived 2008-04-22 at the Wayback Automobile
Source: https://en.wikipedia.org/wiki/Endoplasmic_reticulum
Posted by: wellshasurseen.blogspot.com

0 Response to "What Is The Function Of The Rough Endoplasmic Reticulum In A Animal Cell"
Post a Comment