Which Statement Accurately Describes Reproduction In Animals?


Sexual reproduction is an adaptive characteristic which is common to almost all multi-cellular organisms (and also some single-cellular organisms) with many being incapable of reproducing asexually. Prior to the advent of sexual reproduction, the adaptation process whereby genes would change from one generation to the next (genetic mutation) happened very slowly and randomly. Sex evolved every bit an extremely efficient mechanism for producing variation, and this had the major advantage of enabling organisms to adapt to changing environments. Sexual practice did, still, come with a toll. In reproducing asexually, no time nor free energy needs to exist expended in choosing a mate. And if the environment has non changed, then there may be piffling reason for variation, equally the organism may already be well adapted. Sexual practice, nonetheless, has evolved as the most prolific means of species branching into the tree of life. Diversification into the phylogenetic tree happens much more than quickly via sexual reproduction than it does by manner of asexual reproduction.
Development of sexual reproduction describes how sexually reproducing animals, plants, fungi and protists could have evolved from a common ancestor that was a single-celled eukaryotic species.[1] [two] [3] Sexual reproduction is widespread in the Eukarya, though a few eukaryotic species accept secondarily lost the ability to reproduce sexually, such equally Bdelloidea, and some plants and animals routinely reproduce asexually (past apomixis and parthenogenesis) without entirely having lost sex. The development of sex contains two related yet distinct themes: its origin and its maintenance.
The origin of sexual reproduction tin can exist traced to early prokaryotes, around ii billion years agone (Gya), when bacteria began exchanging genes via conjugation, transformation, and transduction.[4] Though these processes are distinct from true sexual reproduction, they share some basic similarities. In eukaryotes, true sex is thought to have arisen in the concluding eukaryotic common ancestor, maybe via several processes of varying success, and so to have persisted (compare to "LUCA").[5]
Since hypotheses for the origin of sexual practice are hard to verify experimentally (outside of evolutionary computation), almost current piece of work has focused on the persistence of sexual reproduction over evolutionary time. The maintenance of sexual reproduction (specifically, of its dioecious form) by natural selection in a highly competitive world has long been one of the major mysteries of biology, since both other known mechanisms of reproduction – asexual reproduction and hermaphroditism – possess apparent advantages over it. Asexual reproduction can proceed by budding, fission, or spore formation and does not involve the union of gametes, which accordingly results in a much faster rate of reproduction compared to sexual reproduction, where fifty% of offspring are males and unable to produce offspring themselves. In hermaphroditic reproduction, each of the ii parent organisms required for the formation of a zygote tin provide either the male or the female gamete, which leads to advantages in both size and genetic variance of a population.
Sexual reproduction therefore must offer significant fettle advantages because, despite the 2-fold price of sexual practice (see below), information technology dominates amongst multicellular forms of life, implying that the fettle of offspring produced by sexual processes outweighs the costs. Sexual reproduction derives from recombination, where parent genotypes are reorganized and shared with the offspring. This stands in dissimilarity to single-parent asexual replication, where the offspring is always identical to the parents (barring mutation). Recombination supplies two error-tolerance mechanisms at the molecular level: recombinational Dna repair (promoted during meiosis considering homologous chromosomes pair at that time) and complementation (also known as heterosis, hybrid vigor or masking of mutations).
Historical perspective [edit]
The issue of the evolution of sexual reproduction features in the writings of Aristotle, and modern philosophical-scientific thinking on the problem dates from at least Erasmus Darwin (1731–1802) in the 18th century. August Weismann picked up the thread in 1889, arguing that sex activity serves to generate genetic variation, as detailed in the majority of the explanations below. On the other mitt, Charles Darwin (1809–1882) concluded that the effect of hybrid vigor (complementation) "is amply sufficient to account for the ... genesis of the ii sexes".[ citation needed ] This is consistent with the repair and complementation hypothesis, described below. Since the emergence of the modern evolutionary synthesis in the 20th century, numerous biologists including Westward. D. Hamilton, Alexey Kondrashov, George C. Williams, Harris Bernstein, Carol Bernstein, Michael M. Cox, Frederic A. Hopf and Richard E. Michod – have suggested competing explanations for how a vast assortment of different living species maintain sexual reproduction.
Advantages of sex and sexual reproduction [edit]
The concept of sex includes two cardinal phenomena: the sexual process (fusion of genetic information of 2 individuals) and sexual differentiation (separation of this information into 2 parts). Depending on the presence or absence of these phenomena, all of the existing forms of reproduction tin can be classified every bit asexual, hermaphrodite or dioecious. The sexual procedure and sexual differentiation are dissimilar phenomena, and, in essence, are diametrically opposed. The first creates (increases) diversity of genotypes, and the second decreases it by one-half.
Reproductive advantages of the asexual forms are in quantity of the progeny, and the advantages of the hermaphrodite forms are in maximal diversity. Transition from the hermaphrodite to dioecious land leads to a loss of at least half of the multifariousness. And so, the main claiming is to explain the advantages given by sexual differentiation, i.e. the benefits of two separate sexes compared to hermaphrodites rather than to explain benefits of sexual forms (hermaphrodite + dioecious) over asexual ones. It has already been understood that since sexual reproduction is not associated with any clear reproductive advantages, as compared with asexual, there should be some important advantages in evolution.[half-dozen] [ better source needed ]
Advantages due to genetic variation [edit]
For the advantage due to genetic variation, there are iii possible reasons this might happen. First, sexual reproduction can combine the furnishings of two beneficial mutations in the same individual (i.e. sex aids in the spread of advantageous traits). Also, the necessary mutations do non have to have occurred ane later on some other in a single line of descendants.[vii] [ unreliable source? ] Second, sex acts to bring together currently deleterious mutations to create severely unfit individuals that are then eliminated from the population (i.e. sex aids in the removal of deleterious genes). However, in organisms containing just one set of chromosomes, deleterious mutations would be eliminated immediately, and therefore removal of harmful mutations is an unlikely benefit for sexual reproduction. Lastly, sex creates new factor combinations that may be more fit than previously existing ones, or may simply lead to reduced competition among relatives.
For the advantage due to Dna repair, there is an immediate big do good of removing DNA damage past recombinational DNA repair during meiosis, since this removal allows greater survival of progeny with undamaged Dna. The reward of complementation to each sexual partner is abstention of the bad effects of their deleterious recessive genes in progeny past the masking outcome of normal dominant genes contributed by the other partner.[ citation needed ]
The classes of hypotheses based on the creation of variation are farther cleaved downward below. Whatsoever number of these hypotheses may exist truthful in whatever given species (they are not mutually exclusive), and different hypotheses may apply in unlike species. Notwithstanding, a inquiry framework based on creation of variation has still to be found that allows 1 to determine whether the reason for sex is universal for all sexual species, and, if non, which mechanisms are acting in each species.
On the other hand, the maintenance of sexual activity based on DNA repair and complementation applies widely to all sexual species.
Protection from major genetic mutation [edit]
In contrast to the view that sex promotes genetic variation, Heng,[8] and Gorelick and Heng[9] reviewed evidence that sexual activity actually acts as a constraint on genetic variation. They consider that sexual activity acts as a coarse filter, weeding out major genetic changes, such equally chromosomal rearrangements, merely permitting small-scale variation, such as changes at the nucleotide or cistron level (that are often neutral) to pass through the sexual sieve.
Novel genotypes [edit]
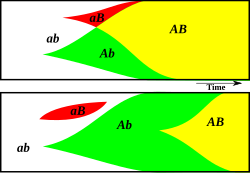
This diagram illustrates how sex might create novel genotypes more rapidly. Two advantageous alleles A and B occur at random. The two alleles are recombined apace in a sexual population (top), but in an asexual population (bottom) the two alleles must independently arise considering of clonal interference.
Sexual activity could be a method by which novel genotypes are created. Because sex combines genes from two individuals, sexually reproducing populations can more easily combine advantageous genes than tin asexual populations. If, in a sexual population, two different advantageous alleles arise at different loci on a chromosome in different members of the population, a chromosome containing the ii advantageous alleles can be produced within a few generations past recombination. However, should the same two alleles arise in unlike members of an asexual population, the but way that i chromosome can develop the other allele is to independently gain the same mutation, which would have much longer. Several studies have addressed counterarguments, and the question of whether this model is sufficiently robust to explain the predominance of sexual versus asexual reproduction remains.[ten] : 73–86
Ronald Fisher besides suggested that sexual activity might facilitate the spread of advantageous genes past allowing them to amend escape their genetic surroundings, if they should arise on a chromosome with deleterious genes.
Supporters of these theories respond to the residue argument that the individuals produced past sexual and asexual reproduction may differ in other respects too – which may influence the persistence of sexuality. For example, in the heterogamous water fleas of the genus Cladocera, sexual offspring form eggs which are better able to survive the winter versus those the fleas produce asexually.
Increased resistance to parasites [edit]
Ane of the most widely discussed theories to explain the persistence of sexual activity is that it is maintained to assist sexual individuals in resisting parasites, also known every bit the Crimson Queen Hypothesis.[eleven] [10] : 113–117 [12] [13] [14]
When an environment changes, previously neutral or deleterious alleles can become favourable. If the surround inverse sufficiently chop-chop (i.due east. between generations), these changes in the environment can make sex advantageous for the individual. Such rapid changes in environs are acquired by the co-evolution between hosts and parasites.
Imagine, for instance that there is ane gene in parasites with 2 alleles p and P conferring 2 types of parasitic power, and ane factor in hosts with two alleles h and H, conferring two types of parasite resistance, such that parasites with allele p can attach themselves to hosts with the allele h, and P to H. Such a state of affairs will lead to cyclic changes in allele frequency – as p increases in frequency, h will exist disfavoured.
In reality, there volition be several genes involved in the human relationship between hosts and parasites. In an asexual population of hosts, offspring will only have the different parasitic resistance if a mutation arises. In a sexual population of hosts, nevertheless, offspring will have a new combination of parasitic resistance alleles.
In other words, like Lewis Carroll'due south Red Queen, sexual hosts are continually "running" (adapting) to "stay in 1 place" (resist parasites).
Evidence for this explanation for the development of sex is provided by comparing of the rate of molecular evolution of genes for kinases and immunoglobulins in the immune system with genes coding other proteins. The genes coding for immune system proteins evolve considerably faster.[15] [16]
Farther evidence for the Red Queen hypothesis was provided past observing long-term dynamics and parasite coevolution in a "mixed" (sexual and asexual) population of snails (Potamopyrgus antipodarum). The number of sexuals, the number asexuals, and the rates of parasite infection for both were monitored. It was establish that clones that were plentiful at the beginning of the study became more than susceptible to parasites over fourth dimension. As parasite infections increased, the once plentiful clones dwindled dramatically in number. Some clonal types disappeared entirely. Meanwhile, sexual snail populations remained much more stable over time.[17] [18]
All the same, Hanley et al.[19] studied mite infestations of a parthenogenetic gecko species and its two related sexual ancestral species. Contrary to expectation based on the Red Queen hypothesis, they found that the prevalence, abundance and mean intensity of mites in sexual geckos was significantly college than in asexuals sharing the same habitat.
In 2011, researchers used the microscopic roundworm Caenorhabditis elegans as a host and the pathogenic leaner Serratia marcescens to generate a host-parasite coevolutionary organization in a controlled environs, assuasive them to comport more than 70 evolution experiments testing the Cherry-red Queen Hypothesis. They genetically manipulated the mating organization of C. elegans, causing populations to mate either sexually, by self-fertilization, or a mixture of both inside the same population. So they exposed those populations to the S. marcescens parasite. It was found that the self-fertilizing populations of C. elegans were rapidly driven extinct past the coevolving parasites while sexual activity allowed populations to keep pace with their parasites, a issue consistent with the Red Queen Hypothesis.[20] [21] In natural populations of C. elegans, self-fertilization is the predominant mode of reproduction, but infrequent out-crossing events occur at a charge per unit of nearly 1%.[22]
Critics of the Ruddy Queen hypothesis question whether the constantly changing environs of hosts and parasites is sufficiently common to explain the evolution of sexual activity. In particular, Otto and Nuismer [23] presented results showing that species interactions (due east.g. host vs parasite interactions) typically select against sex. They concluded that, although the Red Queen hypothesis favors sex activity nether sure circumstances, information technology alone does non account for the ubiquity of sex. Otto and Gerstein [24] further stated that "it seems doubtful to us that strong option per gene is sufficiently commonplace for the Red Queen hypothesis to explicate the ubiquity of sexual activity". Parker[25] reviewed numerous genetic studies on found disease resistance and failed to uncover a single example consistent with the assumptions of the Scarlet Queen hypothesis.
Disadvantages of sex and sexual reproduction [edit]
The paradox of the existence of sexual reproduction is that though information technology is ubiquitous in multicellular organisms, there are ostensibly many inherent disadvantages to reproducing sexually when weighed against the relative advantages of alternative forms of reproduction, such as asexual reproduction. Thus, because sexual reproduction abounds in complex multicellular life, there must be some significant do good(southward) to sex and sexual reproduction that compensates for these fundamental disadvantages.
Population expansion cost of sex [edit]
Amongst the most limiting disadvantages to the evolution of sexual reproduction by natural selection is that an asexual population can grow much more than speedily than a sexual one with each generation.
For case, assume that the entire population of some theoretical species has 100 full organisms consisting of two sexes (i.due east. males and females), with 50:fifty male-to-female person representation, and that only the females of this species can deport offspring. If all capable members of this population procreated once, a total of 50 offspring would be produced (the Fane generation). Contrast this outcome with an asexual species, in which each and every member of an equally sized 100-organism population is capable of bearing immature. If all capable members of this asexual population procreated once, a total of 100 offspring would be produced – twice as many as produced by the sexual population in a single generation.
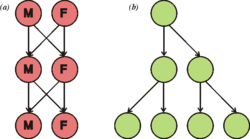
This diagram illustrates the two-fold toll of sex. If each individual were to contribute to the same number of offspring (two), (a) the sexual population remains the same size each generation, where the (b) asexual population doubles in size each generation.
This idea is sometimes referred to as the ii-fold cost of sexual reproduction. It was first described mathematically by John Maynard Smith.[26] [ page needed ] In his manuscript, Smith further speculated on the bear on of an asexual mutant arising in a sexual population, which suppresses meiosis and allows eggs to develop into offspring genetically identical to the mother by mitotic sectionalisation.[27] [ folio needed ] The mutant-asexual lineage would double its representation in the population each generation, all else existence equal.
Technically the problem above is non one of sexual reproduction but of having a subset of organisms incapable of begetting offspring. Indeed, some multicellular organisms (isogamous) engage in sexual reproduction just all members of the species are capable of bearing offspring.[28] [ page needed ] The two-fold reproductive disadvantage assumes that males contribute only genes to their offspring and sexual females waste product half their reproductive potential on sons.[27] [ page needed ] Thus, in this formulation, the principal toll of sex is that males and females must successfully copulate, which most always involves expending energy to come together through time and space. Asexual organisms need not expend the energy necessary to discover a mate.
Selfish cytoplasmic genes [edit]
Sexual reproduction implies that chromosomes and alleles segregate and recombine in every generation, but not all genes are transmitted together to the offspring.[27] [ folio needed ] At that place is a gamble of spreading mutants that cause unfair manual at the expense of their non-mutant colleagues. These mutations are referred to as "selfish" considering they promote their own spread at the cost of culling alleles or of the host organism; they include nuclear meiotic drivers and selfish cytoplasmic genes.[27] [ page needed ] Meiotic drivers are genes that distort meiosis to produce gametes containing themselves more than the 50% of the time expected by chance. A selfish cytoplasmic gene is a gene located in an organelle, plasmid or intracellular parasite that modifies reproduction to crusade its own increase at the expense of the cell or organism that carries it.[27] [ folio needed ]
Genetic heritability cost of sex [edit]
A sexually reproducing organism only passes on ~50% of its ain genetic textile to each L2 offspring. This is a consequence of the fact that gametes from sexually reproducing species are haploid. Once more, however, this is non applicable to all sexual organisms. At that place are numerous species which are sexual but practise not take a genetic-loss problem because they practice non produce males or females. Yeast, for example, are isogamous sexual organisms which have 2 mating types which fuse and recombine their haploid genomes. Both sexes reproduce during the haploid and diploid stages of their life cycle and accept a 100% chance of passing their genes into their offspring.[28] [ page needed ]
Some species avoid the 50% toll of sexual reproduction, although they have "sex" (in the sense of genetic recombination). In these species (e.grand., bacteria, ciliates, dinoflagellates and diatoms), "sex" and reproduction occurs separately.[29] [30]
Deoxyribonucleic acid repair and complementation [edit]
Equally discussed in the earlier function of this article, sexual reproduction is conventionally explained equally an accommodation for producing genetic variation through allelic recombination. Every bit acknowledged in a higher place, however, serious problems with this caption have led many biologists to conclude that the benefit of sexual practice is a major unsolved problem in evolutionary biological science.
An alternative "informational" approach to this problem has led to the view that the two central aspects of sex, genetic recombination and outcrossing, are adaptive responses to the two major sources of "noise" in transmitting genetic information. Genetic dissonance tin occur as either concrete damage to the genome (e.m. chemically altered bases of Deoxyribonucleic acid or breaks in the chromosome) or replication errors (mutations).[31] [32] [33] This alternative view is referred to equally the repair and complementation hypothesis, to distinguish it from the traditional variation hypothesis.
The repair and complementation hypothesis assumes that genetic recombination is fundamentally a DNA repair procedure, and that when it occurs during meiosis information technology is an adaptation for repairing the genomic Deoxyribonucleic acid which is passed on to progeny. Recombinational repair is the just repair process known which can accurately remove double-strand amercement in Dna, and such damages are both mutual in nature and ordinarily lethal if not repaired. For instance, double-strand breaks in DNA occur about 50 times per prison cell cycle in human cells (see naturally occurring DNA impairment). Recombinational repair is prevalent from the simplest viruses to the most complex multicellular eukaryotes. It is effective against many dissimilar types of genomic damage, and in item is highly efficient at overcoming double-strand amercement. Studies of the mechanism of meiotic recombination signal that meiosis is an adaptation for repairing Deoxyribonucleic acid.[34] These considerations class the footing for the first role of the repair and complementation hypothesis.
In some lines of descent from the primeval organisms, the diploid stage of the sexual bike, which was at get-go transient, became the predominant stage, because information technology allowed complementation — the masking of deleterious recessive mutations (i.e. hybrid vigor or heterosis). Outcrossing, the second central aspect of sex activity, is maintained past the reward of masking mutations and the disadvantage of inbreeding (mating with a close relative) which allows expression of recessive mutations (commonly observed as inbreeding depression). This is in accord with Charles Darwin,[35] who concluded that the adaptive advantage of sex is hybrid vigor; or as he put it, "the offspring of two individuals, especially if their progenitors take been subjected to very unlike conditions, accept a bang-up advantage in height, weight, ramble vigor and fertility over the self fertilised offspring from either i of the same parents."
However, outcrossing may be abandoned in favor of parthenogenesis or selfing (which retain the advantage of meiotic recombinational repair) under conditions in which the costs of mating are very high. For example, costs of mating are high when individuals are rare in a geographic area, such as when there has been a forest fire and the individuals entering the burned area are the initial ones to arrive. At such times mates are hard to find, and this favors parthenogenic species.
In the view of the repair and complementation hypothesis, the removal of Dna damage by recombinational repair produces a new, less deleterious form of informational noise, allelic recombination, as a by-product. This lesser advisory noise generates genetic variation, viewed by some every bit the major outcome of sex, as discussed in the earlier parts of this article.
Deleterious mutation clearance [edit]
Mutations tin have many different effects upon an organism. It is generally believed that the majority of non-neutral mutations are deleterious, which means that they will cause a decrease in the organism's overall fitness.[36] [ page range besides broad ] If a mutation has a deleterious consequence, it volition then ordinarily exist removed from the population by the process of natural choice. Sexual reproduction is believed to be more than efficient than asexual reproduction in removing those mutations from the genome.[37]
There are 2 chief hypotheses which explicate how sex may act to remove deleterious genes from the genome.
Evading harmful mutation build-up [edit]
While DNA is able to recombine to modify alleles, Deoxyribonucleic acid is also susceptible to mutations within the sequence that can affect an organism in a negative fashion. Asexual organisms do not have the ability to recombine their genetic information to grade new and differing alleles. Once a mutation occurs in the DNA or other genetic carrying sequence, in that location is no mode for the mutation to be removed from the population until another mutation occurs that ultimately deletes the main mutation. This is rare among organisms.
Hermann Joseph Muller introduced the idea that mutations build up in asexual reproducing organisms. Muller described this occurrence by comparison the mutations that accrue as a ratchet. Each mutation that arises in asexually reproducing organisms turns the ratchet once. The ratchet is unable to be rotated backwards, but forwards. The next mutation that occurs turns the ratchet over again. Boosted mutations in a population continually plow the ratchet and the mutations, mostly deleterious, continually accumulate without recombination.[38] These mutations are passed onto the next generation considering the offspring are verbal genetic clones of their parents. The genetic load of organisms and their populations will increase due to the addition of multiple deleterious mutations and decrease the overall reproductive success and fitness.
For sexually reproducing populations, studies accept shown that single-celled bottlenecks are beneficial for resisting mutation build-up[ citation needed ]. Passaging a population through a unmarried-celled bottleneck involves the fertilization event occurring with haploid sets of Dna, forming ane fertilized cell. For example, humans undergo a single-celled bottleneck in that the haploid sperm fertilizes the haploid egg, forming the diploid zygote, which is unicellular. This passage through a single jail cell is beneficial in that information technology lowers the gamble of mutations from being passed on through multiple individuals. Instead, the mutation is but passed onto one private.[39] Further studies using Dictyostelium discoideum propose that this unicellular initial phase is important for resisting mutations due to the importance of loftier relatedness. Highly related individuals are more than closely related, and more than clonal, whereas less related individuals are less so, increasing the likelihood that an individual in a population of low relatedness may have a detrimental mutation. Highly related populations also tend to thrive better than lowly related because the cost of sacrificing an private is greatly offset by the do good gained by its relatives and in plough, its genes, according to kin pick. The studies with D. discoideum showed that weather condition of high relatedness resisted mutant individuals more effectively than those of depression relatedness, suggesting the importance of high relatedness to resist mutations from proliferating.[forty]
Removal of deleterious genes [edit]

Diagram illustrating dissimilar relationships between numbers of mutations and fitness. Kondrashov's model requires synergistic epistasis, which is represented by the cherry line[41] [42] – each subsequent mutation has a disproportionately large effect on the organism'due south fitness.
This hypothesis was proposed by Alexey Kondrashov, and is sometimes known equally the deterministic mutation hypothesis.[37] It assumes that the majority of deleterious mutations are only slightly deleterious, and affect the individual such that the introduction of each additional mutation has an increasingly large event on the fitness of the organism. This relationship betwixt number of mutations and fitness is known as synergistic epistasis.
By way of analogy, think of a auto with several small faults. Each is not sufficient lonely to prevent the car from running, but in combination, the faults combine to foreclose the automobile from performance.
Similarly, an organism may exist able to cope with a few defects, but the presence of many mutations could overwhelm its backup mechanisms.
Kondrashov argues that the slightly deleterious nature of mutations means that the population will tend to be composed of individuals with a small number of mutations. Sex will act to recombine these genotypes, creating some individuals with fewer deleterious mutations, and some with more. Considering there is a major selective disadvantage to individuals with more mutations, these individuals dice out. In essence, sex compartmentalises the deleterious mutations.
There has been much criticism of Kondrashov's theory, since it relies on ii central restrictive weather. The get-go requires that the charge per unit of deleterious mutation should exceed one per genome per generation in club to provide a substantial advantage for sex activity. While there is some empirical evidence for it (for example in Drosophila[43] and East. coli[44]), there is too strong bear witness against it. Thus, for case, for the sexual species Saccharomyces cerevisiae (yeast) and Neurospora crassa (mucus), the mutation rate per genome per replication are 0.0027 and 0.0030 respectively. For the nematode worm Caenorhabditis elegans, the mutation charge per unit per effective genome per sexual generation is 0.036.[45] Secondly, there should exist stiff interactions among loci (synergistic epistasis), a mutation-fitness relation for which there is only limited evidence.[46] Conversely, there is also the same amount of show that mutations testify no epistasis (purely additive model) or antagonistic interactions (each additional mutation has a disproportionally minor effect).
Other explanations [edit]
Geodakyan's evolutionary theory of sexual practice [edit]
Geodakyan suggested that sexual dimorphism provides a partition of a species' phenotypes into at least two functional partitions: a female person partition that secures beneficial features of the species and a male person partition that emerged in species with more variable and unpredictable environments. The male partition is suggested to be an "experimental" office of the species that allows the species to expand their ecological niche, and to have alternative configurations. This theory underlines the college variability and higher mortality in males, in comparison to females. This functional partition likewise explains the higher susceptibility to illness in males, in comparing to females and therefore includes the idea of "protection confronting parasites" equally another functionality of male sex. Geodakyan's evolutionary theory of sex was adult in Russia in 1960–1980 and was not known to the Westward till the era of the Internet. Trofimova, who analysed psychological sexual practice differences, hypothesised that the male sex might too provide a "redundancy pruning" function.[47]
Speed of evolution [edit]
Ilan Eshel suggested that sex prevents rapid evolution. He suggests that recombination breaks up favourable gene combinations more often than information technology creates them, and sex is maintained because it ensures pick is longer-term than in asexual populations – so the population is less afflicted by short-term changes.[10] : 85–86 [48] This explanation is not widely accepted, every bit its assumptions are very restrictive.
It has recently been shown in experiments with Chlamydomonas algae that sex can remove the speed limit[ clarification needed ] on evolution.[49]
An data theoretic analysis using a simplified but useful model shows that in asexual reproduction, the information gain per generation of a species is express to 1 fleck per generation, while in sexual reproduction, the information gain is divisional by , where is the size of the genome in bits.[50]
Libertine bubble theory [edit]
The evolution of sexual activity can alternatively be described as a kind of gene exchange that is independent from reproduction.[51] According to the Thierry Lodé'south "libertine chimera theory", sex originated from an archaic gene transfer process amid prebiotic bubbles.[52] [53] The contact among the pre-biotic bubbles could, through simple food or parasitic reactions, promote the transfer of genetic material from one bubble to another. That interactions betwixt two organisms be in balance announced to exist a sufficient status to make these interactions evolutionarily efficient, i.due east. to select bubbling that tolerate these interactions ("libertine" bubbles) through a blind evolutionary process of self-reinforcing factor correlations and compatibility.[54]
The "libertine chimera theory" proposes that meiotic sex evolved in proto-eukaryotes to solve a trouble that bacteria did not accept, namely a large amount of Deoxyribonucleic acid material, occurring in an archaic step of proto-cell germination and genetic exchanges. And so that, rather than providing selective advantages through reproduction, sex activity could be thought of as a series of separate events which combines pace-past-pace some very weak benefits of recombination, meiosis, gametogenesis and syngamy.[55] Therefore, current sexual species could exist descendants of primitive organisms that practiced more stable exchanges in the long term, while asexual species take emerged, much more recently in evolutionary history, from the disharmonize of interest resulting from anisogamy.[ clarification needed ]
Parasites and Muller'southward ratchet
R. Stephen Howard and Curtis Lively were the commencement to advise that the combined effects of parasitism and mutation accumulation tin pb to an increased advantage to sex nether conditions not otherwise predicted (Nature, 1994). Using calculator simulations, they showed that when the two mechanisms act simultaneously the reward to sex over asexual reproduction is larger than for either gene operating alone.
Origin of sexual reproduction [edit]
Many protists reproduce sexually, equally do many multicellular plants, animals, and fungi. In the eukaryotic fossil record, sexual reproduction kickoff appeared nigh ii.0 billion years ago in the Proterozoic Eon,[56] [57] although a later date, i.ii billion years agone, has also been presented.[58] [59] Nonetheless, all sexually reproducing eukaryotic organisms likely derive from a unmarried-celled mutual ancestor.[1] [60] [52] It is likely that the evolution of sex was an integral function of the development of the showtime eukaryotic cell.[61] [62] There are a few species which have secondarily lost this characteristic, such as Bdelloidea and some parthenocarpic plants.
Diploidy [edit]
Organisms need to replicate their genetic material in an efficient and reliable manner. The necessity to repair genetic harm is one of the leading theories explaining the origin of sexual reproduction. Diploid individuals can repair a damaged section of their DNA via homologous recombination, since there are 2 copies of the cistron in the cell and if one copy is damaged, the other re-create is unlikely to be damaged at the same site.
A harmful mutation in a haploid individual, on the other hand, is more than probable to get stock-still (i.e. permanent), since whatsoever Dna repair machinery would accept no source from which to recover the original undamaged sequence.[31] The most primitive class of sex may accept been one organism with damaged Deoxyribonucleic acid replicating an undamaged strand from a similar organism in guild to repair itself.[63]
Meiosis [edit]
If, as show indicates, sexual reproduction arose very early in eukaryotic evolution, the essential features of meiosis may take already been present in the prokaryotic ancestors of eukaryotes.[60] [64] In extant organisms, proteins with central functions in meiosis are similar to fundamental proteins in natural transformation in bacteria and DNA transfer in archaea.[64] [65] For example, recA recombinase, that catalyses the key functions of DNA homology search and strand substitution in the bacterial sexual process of transformation, has orthologs in eukaryotes that perform like functions in meiotic recombination[64] (run into Wikipedia articles RecA, RAD51 and DMC1).
Natural transformation in bacteria, DNA transfer in archaea, and meiosis in eukaryotic microorganisms are induced by stressful circumstances such every bit overcrowding, resources depletion, and DNA dissentious conditions.[54] [64] [65] This suggests that these sexual processes are adaptations for dealing with stress, particularly stress that causes DNA damage. In bacteria, these stresses induce an contradistinct physiologic state, termed competence, that allows active take-up of Dna from a donor bacterium and the integration of this DNA into the recipient genome (run into Natural competence) allowing recombinational repair of the recipients' damaged Dna.[66]
If environmental stresses leading to Dna damage were a persistent claiming to the survival of early microorganisms, then pick would probable accept been continuous through the prokaryote to eukaryote transition,[55] [64] and adaptative adjustments would take followed a class in which bacterial transformation or archaeal Deoxyribonucleic acid transfer naturally gave rise to sexual reproduction in eukaryotes.
Virus-like RNA-based origin [edit]
Sexual activity might also accept been present even earlier, in the hypothesized RNA world that preceded DNA cellular life forms.[67] 1 proposed origin of sex in the RNA earth was based on the blazon of sexual interaction that is known to occur in extant unmarried-stranded segmented RNA viruses, such every bit influenza virus, and in extant double-stranded segmented RNA viruses such as reovirus.[68]
Exposure to conditions that cause RNA damage could have led to blockage of replication and death of these early RNA life forms. Sex would take allowed re-array of segments between two individuals with damaged RNA, permitting undamaged combinations of RNA segments to come together, thus assuasive survival. Such a regeneration phenomenon, known as multiplicity reactivation, occurs in flu virus[69] and reovirus.[70]
Parasitic Dna elements [edit]
Another theory is that sexual reproduction originated from selfish parasitic genetic elements that exchange genetic material (that is: copies of their own genome) for their transmission and propagation. In some organisms, sexual reproduction has been shown to enhance the spread of parasitic genetic elements (eastward.g. yeast, filamentous fungi).[71]
Bacterial conjugation is a course of genetic exchange that some sources describe as "sex", merely technically is non a grade of reproduction, even though information technology is a form of horizontal cistron transfer. However, information technology does support the "selfish factor" part theory, since the factor itself is propagated through the F-plasmid.[63]
A similar origin of sexual reproduction is proposed to take evolved in ancient haloarchaea every bit a combination of ii independent processes: jumping genes and plasmid swapping.[72]
Fractional predation [edit]
A third theory is that sexual activity evolved equally a class of cannibalism: One primitive organism ate some other one, but instead of completely digesting it, some of the eaten organism'south DNA was incorporated into the DNA of the eater.[63] [61]
Vaccination-similar procedure [edit]
Sex activity may also be derived from some other prokaryotic process. A comprehensive theory called "origin of sex as vaccination" proposes that eukaryan sex activity-equally-syngamy (fusion sexual practice) arose from prokaryan unilateral sex activity-as-infection, when infected hosts began swapping nuclearised genomes containing coevolved, vertically transmitted symbionts that provided protection against horizontal superinfection by other, more than virulent symbionts.
Consequently, sex-as-meiosis (fission sex) would evolve as a host strategy for uncoupling from (and thereby render impotent) the acquired symbiotic/parasitic genes.[73]
Mechanistic origin of sexual reproduction [edit]
While theories positing fettle benefits that led to the origin of sexual practice are often problematic,[ citation needed ] several theories addressing the emergence of the mechanisms of sexual reproduction accept been proposed.
Viral eukaryogenesis [edit]
The viral eukaryogenesis (VE) theory proposes that eukaryotic cells arose from a combination of a lysogenic virus, an archaean, and a bacterium. This model suggests that the nucleus originated when the lysogenic virus incorporated genetic material from the archaean and the bacterium and took over the role of data storage for the amalgam. The archaeal host transferred much of its functional genome to the virus during the development of cytoplasm, but retained the role of gene translation and full general metabolism. The bacterium transferred virtually of its functional genome to the virus as it transitioned into a mitochondrion.[74]
For these transformations to lead to the eukaryotic cell cycle, the VE hypothesis specifies a pox-similar virus every bit the lysogenic virus. A pox-like virus is a likely antecedent because of its fundamental similarities with eukaryotic nuclei. These include a double stranded Dna genome, a linear chromosome with brusque telomeric repeats, a complex membrane spring capsid, the ability to produce capped mRNA, and the ability to export the capped mRNA across the viral membrane into the cytoplasm. The presence of a lysogenic pox-like virus antecedent explains the evolution of meiotic sectionalization, an essential component of sexual reproduction.[75]
Meiotic partitioning in the VE hypothesis arose because of the evolutionary pressures placed on the lysogenic virus equally a event of its inability to enter into the lytic cycle. This selective pressure resulted in the evolution of processes assuasive the viruses to spread horizontally throughout the population. The outcome of this selection was prison cell-to-cell fusion. (This is singled-out from the conjugation methods used by bacterial plasmids under evolutionary force per unit area, with of import consequences.)[74] The possibility of this kind of fusion is supported by the presence of fusion proteins in the envelopes of the pox viruses that allow them to fuse with host membranes. These proteins could have been transferred to the prison cell membrane during viral reproduction, enabling cell-to-cell fusion between the virus host and an uninfected jail cell. The theory proposes meiosis originated from the fusion between ii cells infected with related just different viruses which recognised each other as uninfected. Later on the fusion of the 2 cells, incompatibilities betwixt the two viruses result in a meiotic-like cell division.[75]
The two viruses established in the prison cell would initiate replication in response to signals from the host cell. A mitosis-similar jail cell cycle would proceed until the viral membranes dissolved, at which point linear chromosomes would be spring together with centromeres. The homologous nature of the two viral centromeres would incite the grouping of both sets into tetrads. Information technology is speculated that this group may exist the origin of crossing over, characteristic of the first division in mod meiosis. The partitioning apparatus of the mitotic-like prison cell wheel the cells used to replicate independently would then pull each set up of chromosomes to ane side of the cell, still leap by centromeres. These centromeres would preclude their replication in subsequent sectionalisation, resulting in four daughter cells with one re-create of ane of the ii original pox-like viruses. The procedure resulting from combination of 2 similar pox viruses inside the same host closely mimics meiosis.[75]
Neomuran revolution [edit]
An alternative theory, proposed by Thomas Cavalier-Smith, was labeled the Neomuran revolution. The designation "Neomuran revolution" refers to the appearances of the common ancestors of eukaryotes and archaea. Condescending-Smith proposes that the first neomurans emerged 850 1000000 years ago. Other molecular biologists assume that this group appeared much before, but Cavalier-Smith dismisses these claims because they are based on the "theoretically and empirically" unsound model of molecular clocks. Condescending-Smith's theory of the Neomuran revolution has implications for the evolutionary history of the cellular machinery for recombination and sex. Information technology suggests that this machinery evolved in two distinct bouts separated past a long flow of stasis; showtime the appearance of recombination machinery in a bacterial ancestor which was maintained for 3 Gy,[ clarification needed ] until the neomuran revolution when the mechanics were adapted to the presence of nucleosomes. The archaeal products of the revolution maintained recombination machinery that was substantially bacterial, whereas the eukaryotic products broke with this bacterial continuity. They introduced cell fusion and ploidy cycles into cell life histories. Cavalier-Smith argues that both bouts of mechanical evolution were motivated by similar selective forces: the need for accurate DNA replication without loss of viability.[76]
Questions [edit]
Some questions biologists have attempted to answer include:
- Why does sexual reproduction exist, if in many organisms it has a fifty% toll (fettle disadvantage) in relation to asexual reproduction?[29]
- Did mating types (types of gametes, according to their compatibility) arise as a result of anisogamy (gamete dimorphism), or did mating types evolve before anisogamy?[77] [78]
- Why do nearly sexual organisms use a binary mating organization? Grouping itself offers a survival advantage. A binary recognition based arrangement is the most simple and effective method in maintaining species grouping.[79]
- Why do some organisms have gamete dimorphism?
References [edit]
- ^ a b Letunic, I; Bork, P (2006). "Interactive Tree of Life". Retrieved 23 July 2011.
- ^ mLetunic, I; Bork, P (2007). "Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation" (PDF). Bioinformatics. 23 (1): 127–8. doi:x.1093/bioinformatics/btl529. PMID 17050570.
- ^ Letunic, I; Bork, P (2011). "Interactive Tree of Life v2: Online annotation and display of phylogenetic trees fabricated like shooting fish in a barrel" (PDF). Nucleic Acids Research. 39 (Spider web Server outcome): W475–8. doi:10.1093/nar/gkr201. PMC3125724. PMID 21470960.
- ^ Otto, Sarah (2014). "Sexual Reproduction and the Evolution of Sex". Scitable . Retrieved 28 February 2019.
- ^ Goodenough, U.; Heitman, J. (one March 2014). "Origins of Eukaryotic Sexual Reproduction". Common cold Spring Harbor Perspectives in Biology. 6 (three): a016154. doi:ten.1101/cshperspect.a016154. ISSN 1943-0264. PMC3949356. PMID 24591519.
- ^ Crow J.F. (1994). Advantages of Sexual Reproduction, Dev. Gen., vol.xv, pp. 205-213.
- ^ Goldstein, R N (2010). 36 Arguments for the Existence of God: A Work of Fiction. Pantheon. ISBN978-0-307-37818-7.
- ^ Heng HH; Heng, Henry H.Q. (2007). "Elimination of altered karyotypes by sexual reproduction preserves species identity". Genome. 50 (five): 517–524. doi:x.1139/g07-039. PMID 17612621.
- ^ Gorelick R, Heng HH; Heng (2011). "Sex reduces genetic variation: a multidisciplinary review". Evolution. 65 (four): 1088–1098. doi:x.1111/j.1558-5646.2010.01173.x. PMID 21091466.
- ^ a b c Birdsell, JA; Wills, C (2003). The evolutionary origin and maintenance of sexual recombination: A review of contemporary models. Evolutionary Biology. Vol. 33. pp. 27–137. doi:10.1007/978-1-4757-5190-1_2. ISBN978-1-4419-3385-0.
- ^ Matt Ridley 1995 The Blood-red Queen: Sex and the Evolution of Human being Nature 1995 Penguin.
- ^ MacIntyre, Ross J.; Clegg, Michael, T (Eds.), Springer. Hardcover ISBN 978-0306472619, ISBN 0306472619 Softcover ISBN 978-ane-4419-3385-0.
- ^ Van Valen, Fifty. (1973). "A New Evolutionary Police force". Evolutionary Theory. 1: 1–thirty.
- ^ Hamilton, Westward. D.; Axelrod, R.; Tanese, R. (1990). "Sexual reproduction every bit an adaptation to resist parasites". Proceedings of the National Academy of Sciences. 87 (9): 3566–3573. Bibcode:1990PNAS...87.3566H. doi:ten.1073/pnas.87.9.3566. PMC53943. PMID 2185476.
- ^ Kuma, K.; Iwabe, N.; Miyata, T. (1995). "Functional constraints against variations on molecules from the tissue-level - slowly evolving encephalon-specific genes demonstrated by protein-kinase and immunoglobulin supergene families". Molecular Biology and Evolution. 12 (i): 123–130. doi:10.1093/oxfordjournals.molbev.a040181. PMID 7877487.
- ^ Wolfe KH, Sharp PM; Sharp (1993). "Mammalian cistron evolution - nucleotide-sequence deviation betwixt mouse and rat". Periodical of Molecular Evolution. 37 (iv): 441–456. Bibcode:1993JMolE..37..441W. doi:ten.1007/BF00178874. PMID 8308912. S2CID 10437152.
- ^ Jokela, Jukka; Dybdahl, Mark; Lively, Curtis (2009). "The Maintenance of Sexual practice, Clonal Dynamics, and Host-Parasite Coevolution in a Mixed Population of Sexual and Asexual Snails". The American Naturalist. 174 (s1): S43–53. doi:10.1086/599080. JSTOR ten.1086/599080. PMID 19441961. S2CID 6797643.
- ^ "Parasites May Have Had Part In Development Of Sex". Science Daily. 31 July 2009. Retrieved 19 September 2011.
- ^ Hanley KA; Fisher RN; Case TJ (1995). "Lower mite infestations in an asexual gecko compared with its sexual ancestors". Development. 49 (3): 418–426. doi:10.2307/2410266. JSTOR 2410266. PMID 28565091.
- ^ Morran, Levi T.; Schmidt, Olivia G.; Gelarden, Ian A.; Parrish Rc, Raymond C.; Lively, Curtis 1000. (2011). "Running with the Blood-red Queen: Host-Parasite Coevolution Selects for Biparental Sex". Science. 333 (6039): 216–218. Bibcode:2011Sci...333..216M. doi:ten.1126/science.1206360. PMC3402160. PMID 21737739.
- ^ "Sex activity -- Equally We Know It -- Works Cheers to Ever-Evolving Host-Parasite Relationships, Biologists Find". Science Daily. 9 July 2011. Retrieved nineteen September 2011.
- ^ Barrière A, Félix MA (July 2005). "High local genetic diverseness and low outcrossing rate in Caenorhabditis elegans natural populations". Curr. Biol. xv (13): 1176–84. arXiv:q-bio/0508003. Bibcode:2005q.bio.....8003B. doi:10.1016/j.cub.2005.06.022. PMID 16005289. S2CID 2229622.
- ^ Otto SP, Nuismer SL; Nuismer (2004). "Species interactions and the development of sexual practice". Science. 304 (5673): 1018–1020. Bibcode:2004Sci...304.1018O. doi:x.1126/scientific discipline.1094072. PMID 15143283. S2CID 8599387.
- ^ Otto SP, Gerstein AC; Gerstein (August 2006). "Why have sex? The population genetics of sex and recombination". Biochemical Society Transactions. 34 (Pt 4): 519–22. doi:10.1042/BST0340519. PMID 16856849.
- ^ Parker MA (1994). "Pathogens and sex in plants". Evolutionary Ecology. 8 (5): 560–584. doi:ten.1007/BF01238258. S2CID 31756267.
- ^ Smith, J. Maynard (1978). The Evolution of Sex . Cambridge University Press. ISBN9780521293020.
- ^ a b c d east Stearns, S. C. (2005). Evolution : an introduction. Hoekstra, Rolf F. (2nd ed.). Oxford [England]: Oxford University Printing. ISBN978-0199255634. OCLC 56964580.
- ^ a b Hoekstra, Rolf F. (1987). "The Evolution of Sexes". In Stearns, Stephen C. (ed.). The Evolution of Sex activity and its Consequences. Springer Basel AG. ISBN9783034862738.
- ^ a b Ridley, Marking (2003). Evolution (3rd ed.). Wiley. p. 314. ISBN9781405103459.
- ^ Beukeboom, L. & Perrin, N. (2014). The Evolution of Sex Determination. Oxford Academy Printing, p. 5–vi [1]. Online resource, [2].
- ^ a b Bernstein H; Byerly HC; Hopf FA; Michod RE (1984). "Origin of sex". J. Theor. Biol. 110 (3): 323–51. Bibcode:1984JThBi.110..323B. doi:ten.1016/S0022-5193(84)80178-ii. PMID 6209512.
- ^ Bernstein H; Byerly HC; Hopf FA; Michod RE (1985). "Genetic damage, mutation, and the development of sex". Science. 229 (4719): 1277–81. Bibcode:1985Sci...229.1277B. doi:10.1126/science.3898363. PMID 3898363.
- ^ Bernstein H; Hopf FA; Michod RE (1987). The Molecular Basis of the Evolution of Sex. Adv. Genet. Advances in Genetics. Vol. 24. pp. 323–70. doi:x.1016/S0065-2660(08)60012-7. ISBN9780120176243. PMID 3324702.
- ^ Cox MM (2001). "Historical overview: searching for replication help in all of the rec places". Proc. Natl. Acad. Sci. UsaA. 98 (15): 8173–80. Bibcode:2001PNAS...98.8173C. doi:10.1073/pnas.131004998. PMC37418. PMID 11459950.
- ^ Darwin CR (1876). The furnishings of cross and self fertilisation in the vegetable kingdom. London: John Murray. [iii] see page 462
- ^ Griffiths et al. 1999. Factor mutations, p197-234, in Modern Genetic Analysis, New York, Westward.H. Freeman and Company.
- ^ a b Kondrashov, A. S. (1988). "Deleterious mutations and the evolution of sexual reproduction". Nature. 336 (6198): 435–440. Bibcode:1988Natur.336..435K. doi:ten.1038/336435a0. PMID 3057385. S2CID 4233528.
- ^ Muller, H.J. (1964). "The Relation of Recombination to Mutational Advance". Mutation Inquiry. i: 2–ix. doi:10.1016/0027-5107(64)90047-8. PMID 14195748.
- ^ Niklas, Karl J. (1 January 2014). "The evolutionary-developmental origins of multicellularity". American Journal of Phytology. 101 (i): 6–25. doi:x.3732/ajb.1300314. ISSN 0002-9122. PMID 24363320.
- ^ Kuzdzal-Fick, Jennie J.; Fox, Sara A.; Strassmann, Joan Due east.; Queller, David C. (xvi Dec 2011). "High Relatedness Is Necessary and Sufficient to Maintain Multicellularity in Dictyostelium". Scientific discipline. 334 (6062): 1548–1551. Bibcode:2011Sci...334.1548K. doi:10.1126/science.1213272. ISSN 0036-8075. PMID 22174251. S2CID 206537272.
- ^ Ridley M (2004) Evolution, 3rd edition. Blackwell Publishing.
- ^ Charlesworth B, Charlesworth D (2010) Elements of Evolutionary Genetics. Roberts and Company Publishers.
- ^ Whitlock, G. C.; Bourguet, D. (2000). "Factors affecting the genetic load in Drosophila: synergistic epistasis and correlations among fitness components" (PDF). Development. 54 (v): 1654–1660. doi:ten.1554/0014-3820(2000)054[1654:fatgli]2.0.co;ii. PMID 11108592.
- ^ Elena, Southward. F.; Lenski, R. E. (1997). "Test of synergistic interactions among deleterious mutations in leaner". Nature. 390 (6658): 395–398. Bibcode:1997Natur.390..395E. doi:10.1038/37108. PMID 9389477. S2CID 205025450.
- ^ Drake JW; Charlesworth B; Charlesworth D; Crow JF (April 1998). "Rates of spontaneous mutation". Genetics. 148 (4): 1667–86. doi:10.1093/genetics/148.4.1667. PMC1460098. PMID 9560386.
- ^ Sohail, G; Vakhrusheva, OA; Sul, JH; Pulit, SL; Francioli, LC; van den Berg, LH; Veldink, JH; de Bakker, PIW; Bazykin, GA; Kondrashov, AS; Sunyaev, SR (2017). "Negative selection in humans and fruit flies involves synergistic epistasis". Science. 356 (6337): 539–542. Bibcode:2017Sci...356..539S. doi:ten.1126/science.aah5238. PMC6200135. PMID 28473589.
- ^ Trofimova, I. (2015). "Do psychological sex differences reflect evolutionary bi-sexual partition?". American Periodical of Psychology. 128 (4): 485–514. doi:ten.5406/amerjpsyc.128.4.0485. PMID 26721176.
- ^ Eshel, I.; Feldman, MW (May 1970). "On the evolutionary effect of recombination". Theoretical Population Biological science. 1 (1): 88–100. doi:10.1016/0040-5809(70)90043-ii. PMID 5527627.
- ^ Colegrave, Northward. (2002). "Sexual practice releases the speed limit on evolution". Nature. 420 (6916): 664–666. Bibcode:2002Natur.420..664C. doi:10.1038/nature01191. hdl:1842/692. PMID 12478292. S2CID 4382757.
- ^ David MacKay (2003). Data Theory, Inference, and Learning Algorithms (PDF). Cambridge: Cambridge University Press. pp. 269–280.
- ^ Lesbarrères D (2011). "Sex or no sex, reproduction is not the question". BioEssays. 33 (eleven): 818. doi:ten.1002/bies.201100105. PMID 22009640. S2CID 46112804.
- ^ a b Lodé, T (2011). "Sex activity is not a solution for reproduction: the libertine bubble theory". BioEssays. 33 (6): 419–422. doi:10.1002/bies.201000125. PMID 21472739.
- ^ Lodé, T (2011). "The origin of sex was interaction, not reproduction (what'due south sex activity actually all about), Large Idea". New Scientist. 212 (2837): 30–31. doi:x.1016/S0262-4079(11)62719-10.
- ^ a b Lodé, T (2012). "Sexual activity and the origin of genetic exchanges". Trends Evol Biol. 4: e1. doi:10.4081/eb.2012.e1.
- ^ a b Lodé, T (2012). "Take sex activity or not ? Lessons from bacteria". Sexual Dev. 6 (6): 325–328. doi:10.1159/000342879. PMID 22986519.
- ^ Otto, Sarah P. (2008). "Sexual Reproduction and the Evolution of Sexual activity". Nature . Retrieved 1 Oct 2021.
- ^ Zimmer, Carl (5 June 2009). "On The Origin Of Sexual Reproduction". Science. Vol. 324. p. 1254. Retrieved i October 2021.
- ^ Butterfield, Nicholas J. (2000). "Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sexual activity, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes". Paleobiology. 26 (iii): 386. doi:x.1666/0094-8373(2000)026<0386:BPNGNS>2.0.CO;2. Retrieved 12 April 2021.
- ^ Cumming, Vivian (4 July 2016). "The Real Reasons Why We Accept Sex activity". BBC News . Retrieved 12 April 2021.
- ^ a b Bernstein H, Bernstein C (2010). "Evolutionary origin of recombination during meiosis". BioScience. 60 (7): 498–505. doi:10.1525/bio.2010.threescore.vii.five. S2CID 86663600.
- ^ a b Ploompuu, T. (1999). Biosüsteemide mälu teooria [Why the eukaryotic cell memory was needed]. Schola Biotheoretica (in Estonian). Vol. XXV. Tartu: Sulemees. pp. 51–56. ISBN978-9985908150. Abstract in English available online: [4]
- ^ Hörandl E, Speijer D (Feb 2018). "How oxygen gave rising to eukaryotic sex". Proc. Biol. Sci. 285 (1872): 20172706. doi:ten.1098/rspb.2017.2706. PMC5829205. PMID 29436502.
- ^ a b c Olivia Judson (2002). Dr. Tatiana's sex advice to all creation. New York: Metropolitan Books. pp. 233–4. ISBN978-0-8050-6331-8.
- ^ a b c d eastward Bernstein, H., Bernstein, C. Evolutionary origin and adaptive role of meiosis. In "Meiosis", Intech Publ (Ballad Bernstein and Harris Bernstein editors), Chapter 3: 41-75 (2013).
- ^ a b Bernstein H, Bernstein C. Sexual advice in archaea, the precursor to meiosis. pp. 103-117 in Witzany, Guenther, ed. (2017). Biocommunication of Archaea. doi:10.1007/978-3-319-65536-nine. ISBN978-3-319-65535-2. S2CID 26593032.
- ^ Michod RE, Wojciechowski MF, Hoelzer MA (1988). "DNA repair and the evolution of transformation in the bacterium Bacillus subtilis". Genetics. 118 (1): 31–39. doi:10.1093/genetics/118.1.31. PMC1203263. PMID 8608929.
- ^ Eigen M, Gardiner W, Schuster P, Winkler-Oswatitsch R (April 1981). "The origin of genetic information". Scientific American. 244 (4): 88–92, 96, et passim. Bibcode:1981SciAm.244d..88E. doi:10.1038/scientificamerican0481-88. PMID 6164094.
- ^ Bernstein H, Byerly HC, Hopf FA, Michod RE (Oct 1984). "Origin of sex". Periodical of Theoretical Biology. 110 (3): 323–351. Bibcode:1984JThBi.110..323B. doi:10.1016/S0022-5193(84)80178-2. PMID 6209512.
- ^ Barry RD (1961). "The multiplication of influenza virus. II. Multiplicity reactivation of ultraviolet irradiated virus". Virology. 14 (four): 398–405. doi:10.1016/0042-6822(61)90330-0. hdl:1885/109240. PMID 13687359.
- ^ McClain ME, Spendlove RS (1966). "Multiplicity reactivation of reovirus particles afterwards exposure to ultraviolet light". J Bacteriol. 92 (5): 1422–1429. doi:10.1128/JB.92.5.1422-1429.1966. PMC276440. PMID 5924273.
- ^ Hickey D (1982). "Selfish Deoxyribonucleic acid: a sexually-transmitted nuclear parasite". Genetics. 101 (3–4): 519–531. doi:10.1093/genetics/101.3-4.519. PMC1201875. PMID 6293914.
- ^ DasSarma, Shiladitya (2007). "Extreme Microbes". American Scientist. 95 (3): 224–231. doi:10.1511/2007.65.224.
- ^ Sterrer W (2002). "On the origin of sexual activity equally vaccination". Periodical of Theoretical Biology. 216 (iv): 387–396. Bibcode:2002JThBi.216..387S. doi:x.1006/jtbi.2002.3008. PMID 12151256.
- ^ a b Bong, PJ (2001). "Viral eukaryogenesis: Was the ancestor of the nucleus a complex Deoxyribonucleic acid virus?". Journal of Molecular Biology. 53 (3): 251–256. Bibcode:2001JMolE..53..251L. doi:x.1007/s002390010215. PMID 11523012. S2CID 20542871.
- ^ a b c Bell, PJ (2006). "Sexual activity and the eukaryotic prison cell cycle is consequent with a viral ancestry for the eukaryotic nucleus". Journal of Theoretical Biological science. 243 (1): 54–63. Bibcode:2006JThBi.243...54B. doi:10.1016/j.jtbi.2006.05.015. PMID 16846615.
- ^ Cavalier-Smith, Thomas (2006). "Cell development and Earth history: Stasis and revolution". Philosophical Transactions of the Regal Society B: Biological Sciences. 361 (1470): 969–1006. doi:10.1098/rstb.2006.1842. PMC1578732. PMID 16754610.
- ^ T. Togashi, P. Cox (Eds.) The Evolution of Anisogamy. Cambridge University Press, Cambridge; 2011, p. 22-29.
- ^ Beukeboom, L. & Perrin, North. (2014). The Evolution of Sex Conclusion. Oxford University Printing, p. 25 [5]. Online resources, [vi].
- ^ Czárán, T.L.; Hoekstra, R.F. (2006). "Development of sexual asymmetry". BMC Evolutionary Biology. iv: 34–46. doi:10.1186/1471-2148-4-34. PMC524165. PMID 15383154.
Further reading [edit]
- Bell, Graham (1982). The masterpiece of nature: the development and genetics of sexuality. Berkeley: University of California Press. ISBN978-0-520-04583-5.
- Bernstein, Carol; Harris Bernstein (1991). Aging, sexual activity, and Dna repair. Boston: Academic Press. ISBN978-0-12-092860-6.
- Hurst, 50.D.; J.R. Peck (1996). "Recent advances in the understanding of the evolution and maintenance of sexual activity". Trends in Environmental and Evolution. 11 (two): 46–52. doi:ten.1016/0169-5347(96)81041-X. PMID 21237760.
- Levin, Bruce R.; Richard E. Michod (1988). The Evolution of sexual practice: an examination of current ideas . Sunderland, Mass: Sinauer Associates. ISBN978-0-87893-459-1.
- Maynard Smith, John (1978). The evolution of sex. Cambridge, United kingdom: Cambridge University Press. ISBN978-0-521-21887-0.
- Michod, Richard E. (1995). Eros and evolution: a natural philosophy of sexual practice. Reading, Mass: Addison-Wesley Pub. Co. ISBN978-0-201-40754-nine.
- "Scientists put sex origin mystery to bed, Wild strawberry research provides evidence on when gender emerges". NBC News . Retrieved 25 November 2008.
- Ridley, Marker (1993). Evolution. Oxford: Blackwell Scientific. ISBN978-0-632-03481-9.
- Ridley, Mark (2000). Mendel'due south demon: cistron justice and the complexity of life. London: Weidenfeld & Nicolson. ISBN978-0-297-64634-1.
- Ridley, Matt (1995). The Red Queen: sex and the evolution of human nature. New York: Penguin Books. ISBN978-0-fourteen-024548-6.
- Szathmáry, Eörs; John Maynard Smith (1995). The Major Transitions in Evolution. Oxford: Westward.H. Freeman Spektrum. ISBN978-0-7167-4525-9.
- Taylor, Timothy (1996). The prehistory of sex: four million years of human sexual culture. New York: Runted Books. ISBN978-0-553-09694-1.
- Williams, George (1975). Sexual activity and development. Princeton, North.J: Princeton University Press. ISBN978-0-691-08147-two.
External links [edit]
- Why Sexual activity is Good
- An essay summarising the different theories, dating from effectually 2001
- http://www.evolocus.com/Textbooks/Geodakian2012.pdf
Source: https://en.wikipedia.org/wiki/Evolution_of_sexual_reproduction
Posted by: wellshasurseen.blogspot.com



0 Response to "Which Statement Accurately Describes Reproduction In Animals?"
Post a Comment